75-YEAR-OLD MALE WITH A 10-YEAR HISTORY OF CHRONIC ITP

Ron*
BACKGROUND
| AGE: |
75
|
| OCCUPATION: |
Veteran, retired railroad worker, part-time farmer
|
| DATE OF DIAGNOSIS: |
September 9, 2008
|
- Type 2 diabetes, controlled with metformin
- Hyperlipidemia, controlled with simvastatin
- AFib with mild compensated heart failure, controlled with lisinopril, metoprolol XL, and warfarin
- Platelet Counts
- Treatment Summary
Ron's Platelet Counts Over Time
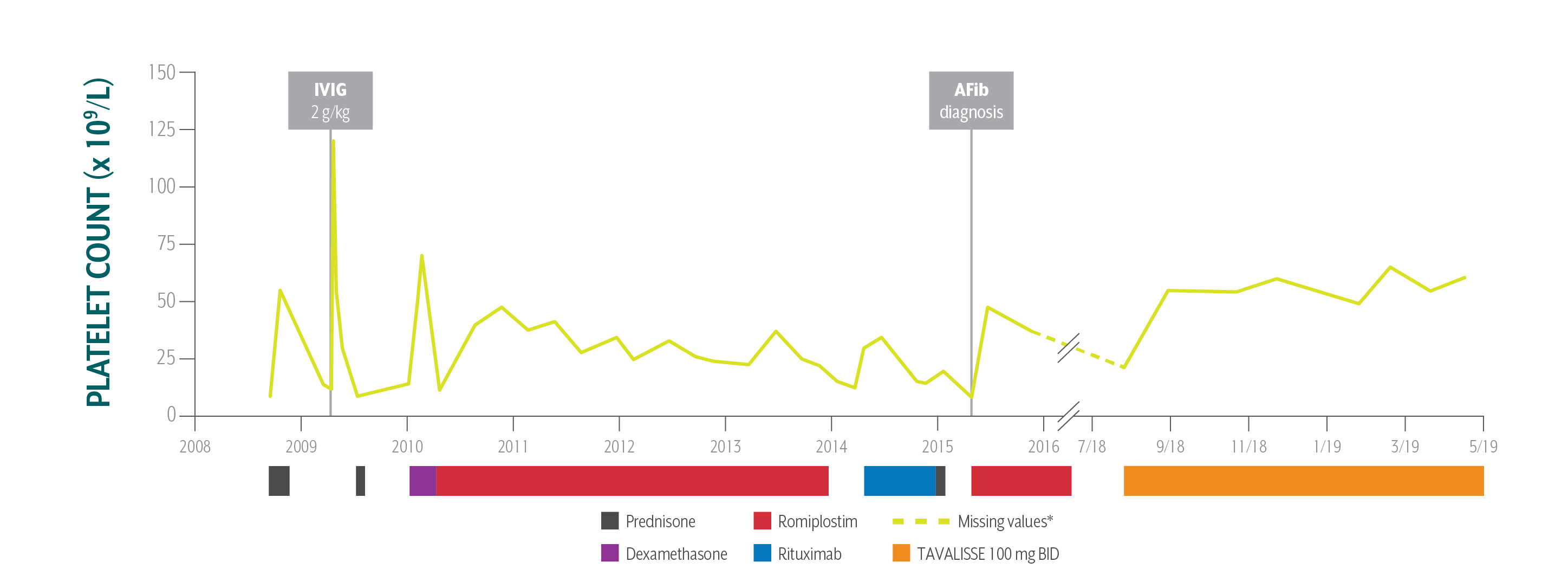
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
Patient responded well to TAVALISSE within 4 weeks; he stabilized his platelet count between 55 x 109/L and 60 x 109/L on the 100 mg BID dose; he has had no bleeding and continues to use warfarin safely.
AFib=atrial fibrillation; ALT=alanine aminotransferase; AST=aspartate aminotransferase; Hb=hemoglobin; IVIG=intravenous immunoglobulin; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Ron’s Treatment Summary
- Headings:
Prior Treatment history
July 9, 2018: Hematology/oncology specialist reviews available medical records
- Headings:
treatment with tavalisse
July 24, 2018: TAVALISSE initiation
- Platelet Count:
9 x 109/L
- Platelet Count:
22 x 109/L
- Clinical Observations:
- Purpura, bruising, epistaxis, fatigue
- Clinical Observations:
- Purpura on lower extremities, petechiae
- Laboratory Findings:
- Normal; comorbidities controlled
- Laboratory Findings:
- Hb: 11.2 g/dL
- WBC: 4.5 x 109/L
- Antiplatelet antibodies: positive
- AST: 32 IU/L
- ALT: 36 IU/L
- Bilirubin: 0.8 mg/dL
- Patient Discussion:
N/A
- Patient Discussion:
- Ron reports fear of injury and bleeding due to active lifestyle as part-time farmer
- Ron is complaining of fatigue
- Ron is adamantly against splenectomy
- Physician explained the mechanism of TAVALISSE and the potential efficacy and adverse events
- Physician counseled Ron on how to manage diarrhea, should it occur
- Treatment Plan:
- Begin treatment with prednisone; discontinue metformin
After learning about Ron’s compliance issues and inconsistent response to prior therapies, the hem-onc sought an oral medication in a different class of therapy.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and increase to 150 mg BID after week 4 if necessary
Patient responded well to TAVALISSE within 4 weeks; he stabilized his platelet count between 55 x 109/L and 60 x 109/L on the 100 mg BID dose; he has had no bleeding and continues to use warfarin safely.
AFib=atrial fibrillation; ALT=alanine aminotransferase; AST=aspartate aminotransferase; Hb=hemoglobin; IVIG=intravenous immunoglobulin; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

PATIENT REFERRED TO NEW HEMATOLOGY/ONCOLOGY SPECIALIST (HEM-ONC) FOR SECOND OPINION AFTER 10 YEARS OF ITP MANAGEMENT